General Science Knowledge
This section of the toolkit will provide an overview of accepted scientific information and principles.
Physical science concepts underpinning OHS Practice

Pryor, P & Capra, M 2012, 'Foundation Science', In HaSPA (Health and Safety Professionals Alliance), The Core Body of Knowledge for Generalist OHS Professionals, Safety Institute of Australia, Tullamarine VIC, p. 4.
Scientific Terms
Absolute Zero: The theoretical lowest temperature possible at which all molecular motion ceases. Absolute zero, 0 K or -273.15°C, has never been reached.
Acceleration: The change in an object's velocity over time, measured in distance per unit time per unit time (for example meters per second per second or m/s2). Acceleration (a) is calculated by dividing the change (symbolized by Δ, the Greek letter delta) in velocity (v) by the change in time (t): a=Δv/ Δt.
Atom: The smallest unit of an element that retains the chemical properties of the element. Atoms can exist alone or in combinations with other atoms forming molecules.
Combustion: Commonly referred to as burning, a chemical reaction between a fuel (for example wood) and an oxidizing agent (for example oxygen) that produces heat (and usually, light).
Density: A measure of the compactness of a substance given by the mass per unit volume (d=m/v). Common units of density include g/ml, g/cm3, and kg/L. A measure of lead is not heavier than an equivalent measure of styrofoam, it is denser.
Endothermic: A process or reaction that absorbs heat. For example, ice melting is an example of an endothermic process because it absorbs heat from its surroundings.
Energy: An abstract property defined as the capacity to do work. The basic forms of energy include chemical, electrical, mechanical, nuclear, and radiant (light).
Exothermic: A process or reaction that releases heat. Wood burning in the presence of oxygen is an example of an exothermic reaction.
First Law of Thermodynamics: The First Law explores the conservation of energy. Specifically, this law explains that energy cannot be created or destroyed, only transformed from one form to another. It also states that the energy within a closed system is fixed – it cannot increase or decrease. The First Law is often expressed as an equation: ∆U=Q - W, or the change in internal energy (∆U) equals the heat added to the system (Q) minus the work done by the system (W).
Force: An influence (a "push or pull") that changes the motion of a moving object (e.g., slows it down, speeds it up, changes its direction) or produces motion in a stationary object. The strength of a force is calculated by multiplying the mass of the object by its acceleration. In the metric (or SI) system, force is measured in newtons.
Frequency: The rate at which a vibration occurs that constitutes a wave, either in a material or in an electromagnetic field, usually measured in hertz (Hz).
Heat: A measure of the total internal energy of a substance that can be increased or decreased when objects with different temperatures are placed into contact. Heat is a process, not a property of a material.
Joule: A metric unit measuring energy or work and named for the British scientist James Prescott Joule. One joule (J) represents the amount of work that can be done by a force of one newton (N) acting over a distance of one meter (m): J=1 N·m or J=1 kg·m2/s2.
Kinetic Energy: The energy an object possesses by virtue of its motion. An object of mass m moving at velocity v has a kinetic energy of ½m·v2.
Mass:A fundamental property of matter which is a numerical measure of the inertia of an object or the amount of matter that an object contains. The mass of an object is different from its weight as mass is independent of the gravitational field exerted on an object.
Newton: A metric unit measuring force and named for English physicist Isaac Newton. One newton (N) represents the force needed to accelerate a one-kilogram (kg) object 1 meter (m) per second (s) per second (s): N=kg·m/s2.
Potential energy: The energy an object possesses by virtue of its position in relation to a field of force. For example, lifting a mass m by h meters increases its potential energy by m·g·h, where g is the acceleration due to gravity.
Second Law of Thermodynamics: The Second Law is commonly referred to as the Law of Increased Entropy. During the conversion of energy from one form to another, some energy is lost to the process of conversion. Though the total energy is in fact conserved, a portion becomes unusable as it increases disorder and randomness within the system.
Third Law of Thermodynamics: The Third Law was developed between 1906 and 1912 by the chemist Walter Nernst and relates specifically to entropy. Nernst identifies that, within a perfect crystal, entropy corresponds to temperature. As the temperature reaches absolute zero, so does the entropy (absolute entropy). Though not physically possible, this defines the mathematical limit of the universe and serves as a reference point for measuring entropy.
Velocity:The speed at which an object is traveling, measured in distance per unit time (for example meters per second or m/s). Compare to acceleration.
Wavelength: The distance between corresponding points on two successive waves, generally measured from crest to crest.
Weight: A measure of the force exerted on an object by a gravitational field. The weight of an object equals its mass times the force of gravity: w=m*g.
Work: A process that occurs when a force acts over a distance, as when an object is moved. Work equals the multiple of the applied force by distance: W=F*d. Work can be thought of as a process in which one form of energy is transformed into another and is commonly expressed in units of joules.
Visionlearning, 2015, Glossary (by discipline), viewed 26 January 2015, http://www.visionlearning.com/en/glossary/discipline/Physics
SI Units - Measurement units
The SI (Système International d'Unités) is a globally agreed system of units, with seven base units which comprise:
-
The ampere (A) - unit of measurement of electric current.
-
The ampere is the unit for electrical current in the International System of Units (SI).
The formal definition of the ampere is that it is the constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed one metre apart in vacuum, would produce between those conductors a force equal to 2 x 10-7 newtons per metre of length.
However the ampere is difficult to realise in practise with sufficient accuracy, so it is realised via the watt (the SI unit for power).
-
-
The kilogram (kg) - unit of measurement of mass
-
The kilogram is the unit for mass. It is the only remaining base unit to be defined by a physical object. All standards of mass must ultimately be traceable to this one object, a cylinder of platinum-iridium alloy kept at the International Bureau of Weights and Measures (BIPM) in France.
-
-
The metre (m) - unit of measurement of length
-
Since 1983, the metre has been internationally defined as the length of the path travelled by light in vacuum during a time interval of 1/ 299 792 458 of a second. This definition can be realised simply and accurately using modern techniques and the speed of light is regarded to be a universal constant, making it ideal as the basis for a length standard.
-
-
The second (s) - unit of measurement of time
-
The second is the duration of 9 192 631 770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium 133 atom
-
-
The kelvin (K) - unit of measurement of thermodynamic temperature
-
The 'standard temperature' we use is the temperature of the triple point of water, which is the unique temperature at which the three phases of water (solid, liquid and vapour) co-exist in equilibrium. We define this temperature to be 273.16 K exactly and hence determine the size of the unit of temperature to be:
The fraction 1/273.16 of the thermodynamic temperature of the triple point of water.
-
-
The mole (mol) - unit of measurement of amount of substance
-
A mole is the amount of substance containing as many elementary entities as there are atoms in exactly 0.012 kilogram (or 12 grams) of carbon-12, where the carbon-12 atoms are unbound, at rest and in their ground state.
The mole is used to describe a practical quantity of material and is the link between the microscopic and macroscopic worlds, used to scale phenomena from the atomic up to 'relevant' sizes. As a result of the definition, the mole contains a defined number of entities, usually atoms or molecules. This number is the Avogadro constant (NA).
-
-
The candela (cd) - unit of measurement of luminous intensity
-
The current definition of the candela was made in 1979, in terms of the watt at only one wavelength of light. It is defined as the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 x 1012 hertz and that has a radiant intensity in that direction of 1/683 watts per steradian (a unit of solid angle).
-
(National Physics Laboratory (NPL), 2014, SI Base Units, viewed 26 January 2015, http://www.npl.co.uk/reference/measurement-units/si-base-units/)
National Physics Laboratory (NPL), 2014, Units of measurement poster, viewed 26 January 2015, http://www.npl.co.uk/upload/pdf/units-of-measurement-poster.pdf
Unit Conversions
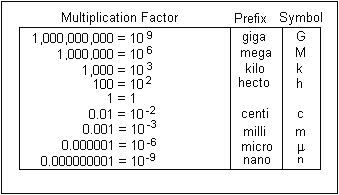
Washington State Department of Transport, 2015, The Metrics International System of Units, viewed 26 January, 2015, http://www.wsdot.wa.gov/reference/metrics/factors.htm.


Washington State Department of Transport, 2015, The Metrics International System of Units, viewed 26 January, 2015, http://www.wsdot.wa.gov/reference/metrics/factors.htm.