Chemical Energy
The Science:
Additional Definitions:
A chemical is “any element, chemical compound, or mixture of elements and/or compounds:”
Element – the simplest form of matter that cannot be broken down further by chemical means. There are currently 109 known elements in the periodic table. Examples of elements are aluminium, carbon, chlorine, hydrogen, mercury and oxygen.
Chemical compound – a substance consisting of two or more elements combined or bonded together so that its constituent elements are always present in the same proportions.
Mixture – any combination of two or more chemicals if the combination is not, in whole or in part, the result of a chemical reaction. (Pisaniello & Tepe, 2012, pp. 11-12).
Reactivity – tendency of a material or combination of materials to undergo chemical change under the right conditions (CCPS, 2001, p. 2)
Energy breaks or makes chemical bonds and depending on its type, heat can be either released (exothermic) or absorbed (endothermic) (see definitions here)
Toxicity
Toxicity refers to the degree to which a substance can damage an organism, in the context of OHS it is how a substance can impact upon a worker. There are five main routes of exposure for workers:
-
Inhalation, through the lungs
-
Absorption, through the skin/mucous membranes
-
Ingestion, orally
-
Injection, into the bloodstream
-
Transfer, pregnant women can transfer exposure from the placenta to the baby. (Tranter 2014 p.8-10)
Applying the energy-based classification of hazards,5 chemical hazards may be defined as those where the potentially hazardous energy is released through disruption of the molecular bonding as a result of chemical reaction (usually a reactive chemical hazard) (Pisaniello & Tepe, 2012, p. 12). Thus, chemical energy is the energy contained within chemical bonds.
The flash point of a chemical is the lowest temperature where it will evaporate enough fluid to form a combustible concentration of gas. The flash point is an indication of how easy a chemical may burn. Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points (The Engineering Toolbox ND, Flash Point, viewed 17 January 2015, http://www.engineeringtoolbox.com/flash-point-d_924.html)
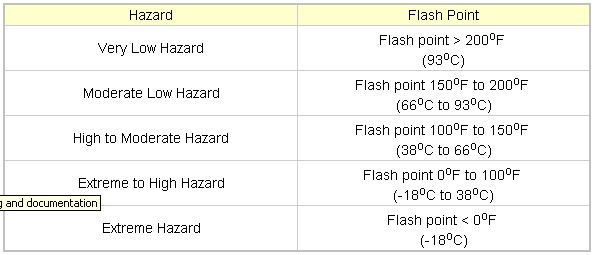
The Engineering Toolbox ND, Flash Point, viewed 17 January 2015, http://www.engineeringtoolbox.com/flash-point-d_924.html
Risk Potential:
To fully comprehend chemical hazards and risks in the workplace, a generalist OHS professional requires knowledge of the toxic and physicochemical attributes of the chemical (the hazard), paired with an understanding of the potential for worker exposure to the chemical or its impacts. This understanding must include factors related to acute and chronic exposure, chemical hazard classification systems, and chemical hazard identification, risk assessment and risk communication.
Acute toxicity, which refers to “the adverse health effects following a single or limited number of exposures” can occur as a result of exposure due to equipment failure, inadequate protection during maintenance or cleaning, or improper handling of a chemical. Although the effects of acute exposures are usually obvious within a short period, the effects of exposure to some chemicals such as pesticides may not appear for several days.
Temporary effects of acute chemical exposure may include: skin irritation, headaches and nausea.
Permanent effects include scars from acid burns.
Chronic toxicity refers to “the adverse health effects resulting from continuous or intermittent exposure” over a prolonged period, often to relatively low levels of chemical. There may be a latency period of many years before the effects of chronic exposure to a chemical are expressed (Pisaniello & Tepe, 2012, p. 17).
There are a number of occupations where chemical hazards may be present. These may include:
-
Cleaners
-
Truck Drivers
-
Steel works
-
Manufacturing
-
Laboratories
-
Aviation
-
Emergency services
-
Hospitals
Consideration to occupations will need to be made when assessing these hazards in future practice.
And if it all goes wrong?
British Pathé 2011, Hindenburg Disaster Real Footage (1937) [HD], video,27 July, viewed 26 January 2015, http://youtu.be/CgWHbpMVQ1U
Measurement and evaluation:
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides an internationally “uniform way of classifying chemicals, as well as informing chemical users about chemical hazards they may be exposed to”. The GHS covers all hazardous chemical substances, solutions and mixtures of chemicals with the classifications linked to hazard symbols, signal words, hazard and precautionary statements (similar to current risk and safety phrases used in labelling hazardous chemicals), which provide information on safe storage, handling, disposal, personal protection and first aid (Pisaniello & Tepe, 2012, p. 19).
To aid measurement and evaluation, verious practitioners may be employed, including an Occupational Hygienist, Epidemioligist andtoxicoligists.
Reference should be made to the National Exposure Standards.
Risk Control Resources:
National Industrial Chemicals Notification and Assessment Scheme - NICNAS
Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (Third Edition)
Safe Work Australia - Hazardous Substances Information System (HSIS)
Safe Work Australia - Workplace Exposure Standards for Airborne Contaminants
Preparation of safety data sheets for hazardous chemicals Code of Practice 2011 (Qld)
Dangerous Goods Safety Management Regulation 2001 (Qld)
Transport Operations (Road Use Management—Dangerous Goods) Regulation 2008 (Qld)
Department of Transport and Main Roads (Qld) - Dangerous Goods
The Australian Dangerous Goods Code Edition 7.3
Peer Reviewed article: Haberer, K 2013, 'SDS & Chemical Management: Getting Return on Investment', Professional Safety, Vol. 58, Issue 9, pp. 46-47.
What Happened?
An employee of a car dealership was severely burnt while cutting a hole in a steel drum using an angle grinder. The following is a brief analysis of the incident based upon the Energy-Damage Model.
The Incident: Northpoint Toyota fined over oil drum explosion
Hazard control mechanism failure: Failure to eliminate hazard as per remainder of locations
Failure to train and supervise adequately
Failure to identify potential hazard (signage)
Failure to have documented procedure
Energy: Chemical Energy resulting in exothermic reaction of the fuel vapours
Thermal energy from sparks caused by grinder
Deformation energy as a result of the reaction.
Space transfer mechanism: The introduction of heat through the use of the angle grinder
Recipients: 1x 18yo apprentice
An 18yo apprentice was severely burnt after a 44-gallon drum being cut with an angle grinder exploded. The drum contained unleaded fuel which has a flash point of -40*C classifying it as a high to moderate hazard. The lower the flash point, the greater the substances’ flammability which reduces the energy required for ignition, also meaning the substance is more reactive. The introduction of thermal energy through the grinding of the barrel (sparks as well as the friction of the blade) has been sufficient to ignite the fuel vapours and achieve a chemical reaction. The reaction was exothermic, giving off both heat and light, as well as releasing deformation energy. This deformation energy has had molecular bonds react to skin cells resulting in second-degree burns to the apprentices’ chest and forearms
References:
Center for Chemical Process Safety 2001, Reactive Material Hazards, viewed 26 January 2015, http://www.aiche.org/ccps/topics/process-safety-technical-areas/chemical-reactivityhazards/reactive-material-hazards
Pisaniello, D & Tepe, S 2012, ‘Chemical Hazards’, In HaSPA (Health and Safety Professionals Alliance, The Core Body of Knowledge for Generalist OHS Professionals, Safety Institute of Australia, Tullamarine VIC
Tranter, M 2004, Occupational hygiene and risk management, 2nd end., Allen & Unwin, Crowns Nest, NSW.